
Back MDA Bulgarian 3,4-methylendioxyamfetamin Czech 3,4-metylendioksyamfetamin Danish 3,4-Methylendioxyamphetamin German MDA (drogo) Esperanto 3,4-metilendioxianfetamina Spanish 3,4-metyleenidioksiamfetamiini Finnish 3,4-Méthylènedioxyamphétamine French Metilenedioxianfetamina Galician MDA (chimica) Italian
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, sublingual, insufflation, intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP extensively involved) |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.230.706 |
| Chemical and physical data | |
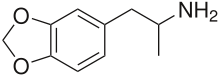
| Formula | C10H13NO2 |
| Molar mass | 179.219 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
3,4-Methylenedioxyamphetamine (also known as MDA and sass) is an empathogen-entactogen, psychostimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.
MDA is rarely sought as a recreational drug compared to other amphetamines; however, it remains widely used due to it being a primary metabolite,[2] the product of hepatic N-dealkylation,[3] of MDMA (ecstasy). It is also a common adulterant of illicitly produced MDMA.[4][5]
- ^ "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control]. Brazilian Health Regulatory Agency (in Brazilian Portuguese). Diário Oficial da União (published 25 July 2023). 24 July 2023. Archived from the original on 27 August 2023. Retrieved 27 August 2023.
- ^ Crean RD, Davis SA, Von Huben SN, Lay CC, Katner SN, Taffe MA (October 2006). "Effects of (+/-)3,4-methylenedioxymethamphetamine, (+/-)3,4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques". Neuroscience. 142 (2): 515–525. doi:10.1016/j.neuroscience.2006.06.033. PMC 1853374. PMID 16876329.
- ^ de la Torre R, Farré M, Roset PN, Pizarro N, Abanades S, Segura M, et al. (April 2004). "Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition". Therapeutic Drug Monitoring. 26 (2): 137–144. doi:10.1097/00007691-200404000-00009. PMID 15228154.
- ^ "EcstasyData.org: Test Result Statistics: Substances by Year". EcstasyData.org. Retrieved 27 June 2017.
- ^ "Trans European Drug Information". idpc.net. Archived from the original on 4 November 2021. Retrieved 27 June 2017.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search